The isotopic shift (also called isotope shift) is the shift in various forms of spectroscopy that occurs when one nuclear isotope is replaced by another.
Atomic spectra
Isotope shifts in atomic spectra are minute differences between the electronic energy levels of different isotopes of the same element. Today they are the focus of a multitude of theoretical and experimental efforts due to their importance for atomic and nuclear physics. If atomic spectra also have hyperfine structure the shift refers to the center of gravity of the spectra.
From a nuclear physics perspective, isotope shifts combine different precise atomic physics probes for studying nuclear structure, and their main use is nuclear-model-independent determination of charge-radii differences.
There are two effects which contribute to this shift:
The mass difference (mass shift), which dominates the isotope shift of light elements[1]. It is traditionally divided to a normal mass shift (NMS) resulting from the change in the reduced electronic mass, and s specific-mass-shift (SMS) which is present in multi-electron atoms and ions. The NMS is a purely kinematical effect, studied theoretically by Hughes and Eckart[2]. The effect of the specific mass shift was first observed in the spectrum of neon isotopes by Nagaoka and Mishima[3].
The volume difference (field shift), which dominates the isotope shift of heavy elements. This difference induces a change in the electric charge distribution of the nucleus. This effect is important in heavy elements and its first theory was formulated by Pauli and Peierls.[4][5][6]. Adopting a simplified picture, the change in an energy level resulting from the volume difference is proportional to the change in total electron probability density at the origin times the mean-square charge radius difference.
NMR spectroscopy
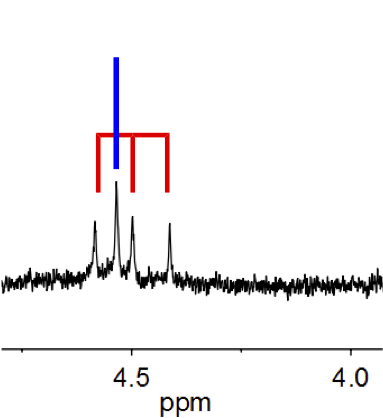
In NMR spectroscopy, Isotopic effects on chemical shifts are typically small, far less than1 ppm the typical unit for measuring shifts. The 1H NMR signals for 1H2 and 1H2H ("HD") are readily distinguished in terms of their chemical shifts. The asymmetry of the signal for the "protio" impurity in CD2Cl2 arises from the differing chemical shifts of CDHCl2 and CH2Cl2.
H NMR spectrum of a solution of HD (labeled with red bars) and H2 (blue bar). The 1:1:1 triplet arises from the coupling of the 1H nucleus (I = 1/2) to the 2H nucleus ( I = 1).
Vibrational spectra
Isotopic shifts are best known and most widely used in vibration spectroscopy where the shifts are large, being proportional to the ratio of the square root of the isotopic masses. In the case of hydrogen, the "H-D shift" is (1/2)1/2 or 1/1.41. Thus, the (totally symmetric) C-H vibration for CH4 and CD4 occur at 2917 cm−1 and 2109 cm−1, respectively.[7] This shift reflects the differing Reduced mass for the affected bonds. See also
Kinetic isotope effect
Magnetic isotope effect
References
King, W. H. (1984), "Isotope Shifts in X-Ray Spectra", Isotope Shifts in Atomic Spectra, Springer US, pp. 55–61, doi:10.1007/978-1-4899-1786-7_5, ISBN 9781489917881
Hughes, D. J.; Eckart, C. (1930). "The Effect of the Motion of the Nucleus on the Spectra of Li I and Li II". Phys. Rev. 36 (4): 694–698. Bibcode:1930PhRv...36..694H. doi:10.1103/PhysRev.36.694.
H. Nagaoka and T. Mishima, Sci. Pap. Inst. Phys. Chem. Res. (Tokyo) 13, 293 (1930).
W. Pauli, R. E. Peierls, Phys. Z. 32 (1931) 670
Brix, P.; Kopfermann, H. (1951). "Neuere Ergebnisse zum Isotopieverschiebungseffekt in den Atomspektren". Festschrift zur Feier des Zweihundertjährigen Bestehens der Akademie der Wissenschaften in Göttingen. Springer. pp. 17–49. doi:10.1007/978-3-642-86703-3_2. ISBN 978-3-540-01540-6.
Kopfermann, H. (1958). Nuclear Moments. Academic Press.
Takehiko Shimanouchi (1972). "Tables of Molecular Vibrational Frequencies Consolidated" (PDF). National Bureau of Standards. NSRDS-NBS-39.
Hellenica World - Scientific Library
Retrieved from "http://en.wikipedia.org/"
All text is available under the terms of the GNU Free Documentation License